With our colleagues, each of whom is an expert in their field, we research, develop, produce and renew to add value to life.
Click here for more...
Career

With our colleagues, each of whom is an expert in their field, we research, develop, produce and renew to add value to life.
Click here for more...

At Bilim Pharmaceuticals, we derive our strength from the talent, determination and courage of our colleagues and offer many opportunities for their development and progress.
Click here for more...

We aim to grow not only with the talents of today, but also with the talents of the future.
Click here for more...

We aim to grow not only with the talents of today, but also with the talents of the future.
Click here for more...
Our Departments that Add Value to Life...
In today's rapidly digitalising business world, Bilim Pharmaceuticals' strategies, in line with its business needs and targets, aim to create a competitive advantage in the markets in which it operates;
In addition, it ensures the continuity of its systems, application platforms, communication and integration infrastructures by determining IT information security policies and standards in accordance with generally accepted standards and regulations (ISO27001, GMAP5, ITIL, KVK/GDPR, PCI DSS, CMMC, etc.) with its expert staff, and ensures the security and cyber resilience of all structures while keeping the confidentiality, integrity and accessibility of data at the highest level.


Bilim Pharmaceuticals' R&D Centre employs more than 100 employees from patent, active substance, new product formulation and analytical development, clinical research, improvement, new product stability and technology groups. In our R&D centre, patent screening and evaluation studies, active substance evaluation, analysis and approval studies, formulation and analytical method development, laboratory and pilot scale productions, process and analytical method validations, clinical studies, product improvement studies, new product stability studies and preparation of registration files in CTD format are carried out with the QbD (Quality by Design) approach.
Today, with the globalisation of trade and production, one of the most important factors of economic development is to increase our exports. Within this framework, Bilim Pharmaceuticals started its export activities in 1998 and today, our products are safely prescribed by doctors in more than 70 countries. Our company, which has the necessary knowledge and experience for international sales and promotion activities with its expert staff, has taken very important steps in recent years towards becoming a global company. Together with our representative offices in Moldova, Albania, Dubai and the teams of our partners, we carry out marketing activities with more than 300 field employees in total.


In order to protect the financial structure of the company and make it stronger, it plans medium and long-term strategies and implements these strategies with a risk management approach.
With our understanding of human resources that adopts adding value to life as a principle, innovative human resources practices are carried out that transform the potential of our employees into performance with sustainable training and learning opportunities and support the development of each employee by recruiting not only today's but also tomorrow's talents.


In addition to adding new products to our portfolio in order to strengthen Bilim Pharmaceuticals' presence in the therapeutic areas in which it operates, the Company continues its activities to evaluate joint ventures, new business partnerships and models that will add value to Bilim Pharmaceuticals and human health by following local and global trends in the pharmaceutical industry.
Technical Services, Occupational Health and Safety and Environmental processes are managed under the Technical General Directorate. In addition to sterile, solid, liquid and pomade production functions, functions supporting the production process such as engineering, maintenance, repair and logistics centre are also carried out within Production Operations. Resource planning and operation management are carried out to ensure efficient production.


Quality Assurance:
It ensures the production of pharmaceutical products in accordance with good manufacturing practices, quality standards and the intended use in accordance with national and international regulations. Bilim Pharmaceuticals Quality Assurance Department, with its specialised staff graduated from the pharmacy, chemistry, biology and chemical engineering departments of universities, guarantees the product efficacy of the products manufactured in its high-tech production facilities throughout the life cycle of the product through the pharmaceutical quality assurance system.
Quality Control:
In accordance with national and international regulations, particularly Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP), all activities that ensure the conformity of starting materials, packaging materials, intermediate products and finished products with approved specifications are carried out with high-tech equipment and electronic systems. The activities carried out in the Quality Control Laboratories are carried out independently from other departments by competent and well-equipped staff graduated from pharmacy, chemistry, biology and chemical engineering departments of universities.
Production Planning Management and Accounting Management departments are located within our Directorate.
Our Production Planning Directorate is responsible for planning supply and production activities and creating stock policies in order to ensure that all products are produced at the desired time, quantity and quality in line with the demands of our customers in domestic and foreign markets.
Our Accounting Management is responsible for general accounting, budget, cost accounting and management accounting processes. In this context, it is responsible for the execution of all legal accounting transactions within the framework of financial legislation, the preparation of the annual budget, and the preparation of declarations and reports requested by internal and external customers.


Within the Marketing Directorate, marketing and sales activities are carried out by product promotion officers in regions, regional managers and promotion managers responsible for the central management of these functions, marketing managers in order to introduce proven scientific data to our doctors, dentists and pharmacists. In addition, other marketing activities of our products are managed by product managers.
In order to meet material, product and service needs in the most appropriate conditions and on time, purchasing activities are carried out in cooperation with our suppliers in line with the determined legislation and procedures.

Continuous development is aimed in parallel with the Company's vision and business targets. The current and future development needs of employees are identified and training programmes are carried out to improve their technical, functional and professional knowledge and skills. Newly recruited employees are supported with orientation programmes that ensure adaptation to the company as well as legally mandatory training.
We see creating a learning organisation as a fundamental element of strategic HR management in order to realise our company's vision. We continue our trainings for the development of our employees' technical, functional, professional knowledge and skills by determining their current and future development needs. For this purpose, we offer new learning methods and different learning tools to all our employees through our bilimk@mpüs platform. We support our newly recruited employees with orientation programmes that ensure adaptation to the company as well as legally mandatory training.
Our Education Programmes;
Orientation
Bilimk@mpüs e-learning platform
Classroom Trainings
Stars Team - Talent Pool Development Programmes
Leadership Development Trainings
Bilim Pharmaceuticals Leader Shares

Career management practices are used as a part of strategic human resources planning in order to meet organisational needs and to evaluate the potential of employees in appropriate positions.
In addition to the job performance of our employees, their transition to different positions is supported through appointment, promotion and rotation processes by using scientifically based assessment tools with proven validity and reliability in line with their competencies and experience. As a result of the evaluations, the development needs of the employees are shared through feedback reports and training and development opportunities are provided for the employees to improve themselves.
The performance management system is implemented with an objective approach in which business results and personal competences are evaluated together. In line with common goals and objectives, our employees are supported to maximise their performance and talents. With this approach, it is aimed to identify the strengths and development needs of our employees and to improve their individual and team performances through regular feedbacks.
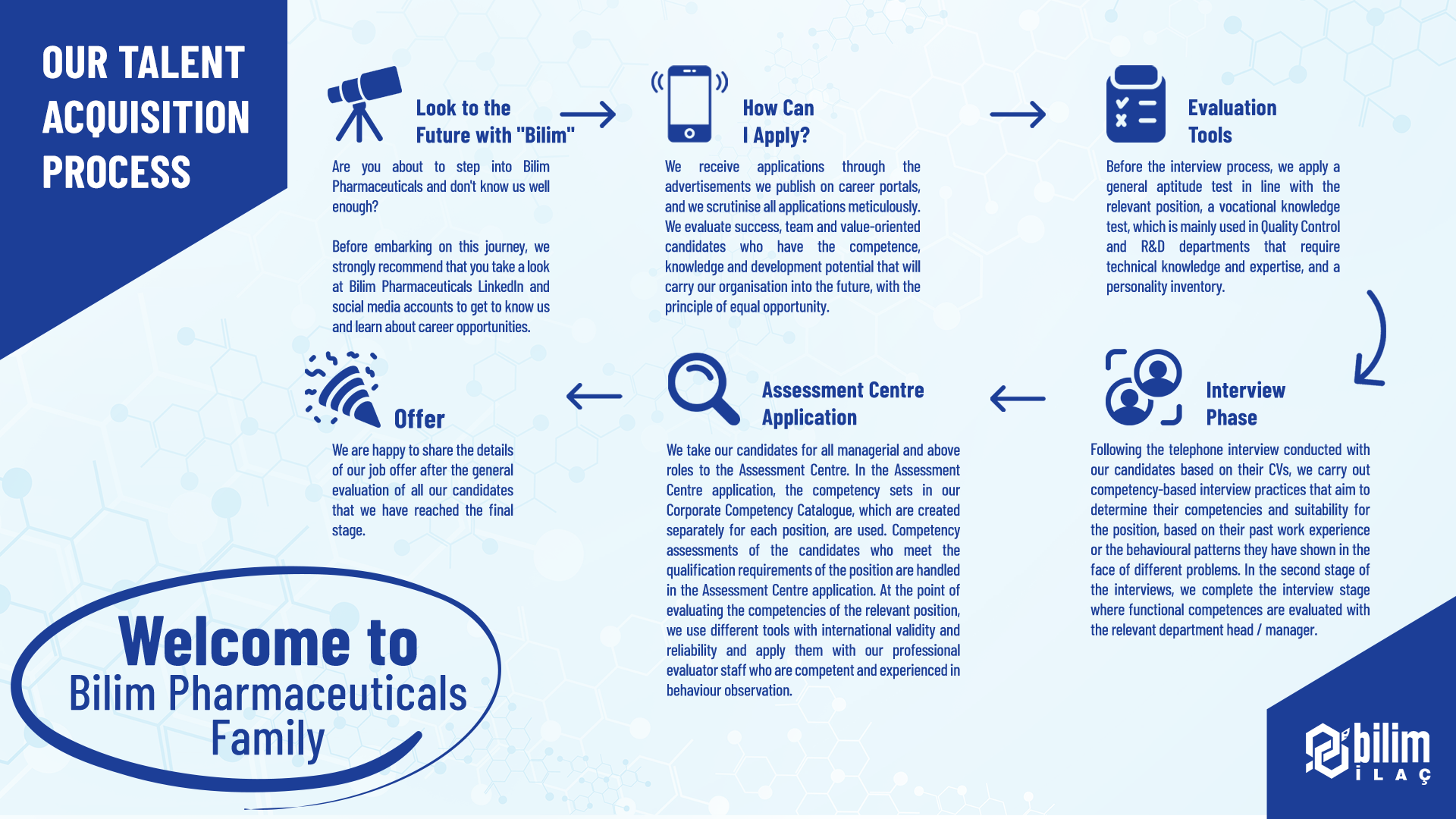

We enable our qualified young talents who continue their undergraduate / associate degree / high school education to work in our departments that add value to life so that they can see the theoretical knowledge they have acquired in practice and get to know business life.
Following the application process, we include our young talents in online general aptitude tests, professional knowledge inventories, foreign language tests and group interview case studies. At the final stage, we aim to provide them with a candidate experience by introducing them to the human resources team and relevant department managers.
We come together with university students by visiting universities to share information about CV preparation, interview experience, Bilim Pharmaceuticals recruitment processes, career opportunities and career choices. We organize meetings where we share information about the job description and career opportunities specific to the relevant department with the conferences we attend together with department managers.
You can follow our advertisements on Kariyer.net and Linkedin to access Bilim Pharmaceuticals job opportunities.
